Abstract
Background. The overall survival (OS) of multiple myeloma (MM) patients (pts) has improved over the years due to the introduction of several novel drugs, such as proteosome inhibitors (PI), immunomodulatory drugs (IMiDs) and, more recently, anti-CD38 monoclonal antibodies (moAb). Nevertheless, the majority of pts continues to relapse, and MM remains an incurable disease. To date, no standard of care has been established for relapsed/refractory (RR) MM pts who have been exposed to the main anti-myeloma drugs. Currently, these pts have a limited number of available treatment options and represent an unmet medical need. Moreover, the outcome of pts failing standard of care regimens, which is now defined as triple-refractory (including PI, IMiDs and moAb), is poor, with a median progression free survival (PFS) of 3-4 months and OS of 8-9 months. Novel therapeutic strategies with different mechanisms of action are warranted to overcome the natural occurrence of relapse or therapy resistance in RR MM pts. Immunotherapy, especially T-cell based approaches, represents the emerging therapeutic strategy for this subset of pts. Chimeric antigen receptor (CAR)-modified T cells are a promising new therapy approach for triple refractory RRMM. Different constructs and specific CAR-T targets are being studied, but BCMA-directed CAR-T cells have so far provided the most convincing evidence of activity, with one product (idecabtage vicleucel) recently approved by FDA.
Aims. The primary endpoint of this observational and retrospective study was to define the clinical characteristics and outcome of a cohort of RR MM pts potentially eligible to CAR-T cell treatment according to the KarMMa trial criteria 1. Secondary endpoints were aimed at defining specific factors influencing CAR-T cell therapy eligibility and at identifying a real-life estimate of RRMM pts truly eligible for CAR-T cells.
Methods and Results. This is a cohort analysis that used electronic REDCap, a data capture tool hosted at the Sapienza University, on RRMM pts managed between January 2018 and July 2021 at 4 Italian Centers of the Multiple Myeloma GIMEMA Lazio Group. At the time of data collection, 47 RRMM pts had underwent at least 3 prior therapy regimens; they had received a previous PI, IMiDs and a moAb and were considered refractory to the last regimen. The clinical characteristics are listed in Table 1. Median age was 68 years (43-86), 27 (61%) pts were >65 years; 27 (57%) were male. Of 47 pts, 33 (68%) were ECOG 0-1 and 14 (30%) were ECOG ³2; 21 pts (44%) were ISS III. The majority of pts, 28 (59%), had undergone an autologous stem cell transplantation; 31 pts (65%) had received 3 prior lines of therapy and 16 (34%) >3 prior lines of therapy. Thirty-seven (78%) were triple-refractory and 8 (17%) were penta-refractory. Based on the KarMMa trial criteria, 22/47 pts (47%) would be defined as eligible and 25 (53%) not eligible for CAR-T cell therapy. Specifically, 14 (30%) pts were not eligible because of an ECOG ³2, 24 (62%) had an organ dysfunction such as impaired renal function (eGFR <45 ml/min/1.73m 2), anemia and thrombocytopenia. Of the 25 pts considered ineligible for CAR-T cell therapy, 17 (68%) presented ≥2 ineligibility criteria. The entire cohort of 47 pts had a negative hepatitis and HIV serology, and no patient had previously undergone anti-BCMA therapy or an allogeneic stem cell transplantation.
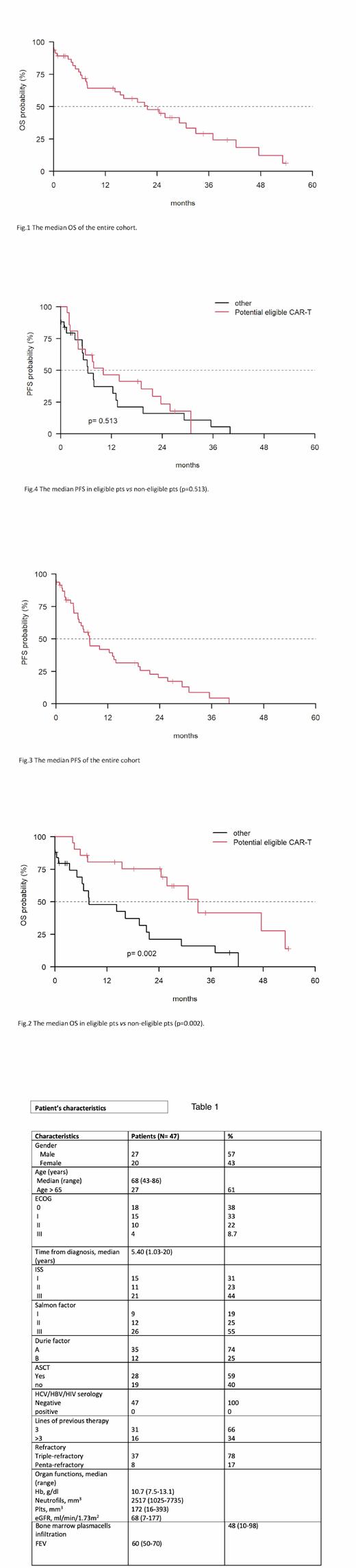
After a median follow-up of 34.7 months (mo) (0-53.8), the median OS for the entire cohort was 21.7 mo (95% CI: 14.2-36.9) (Fig. 1). The median OS was 30.7 mo (95%, CI: 14.21-NA) in eligible pts vs 16.2 mo (95%, CI: 6.28-NA) in non-eligible pts (p=0.002) (Fig.2). The median PFS of the entire cohort was 7.7 mo (95%, CI: 5.39-13.85) (Fig. 3) and the median PFS was 7.8 mo (95%, CI: 5.16-19.1) in eligible pts vs 6.5 mo (95%, CI: 3.36-NA) (p=0.513) in non-eligible pts (Fig.4).
Conclusions. Despite the limits of a retrospective study and a limited cohort, our real-life analysis shows that heavily treated pts with RRMM are less likely to be eligible for CAR-T cell therapy. Considering the emergent role of quadruplet combined approaches for first-line therapy and given the therapeutic relevance of CAR-T cells for the management of RRMM pts previously exposed to PI, IMiDs and moAb, our data could help to better define pts who could benefit from CAR-T cells under the current indications, while waiting for an extension of this approach to earlier disease stages.
1. N Engl J Med 2021;384:705-16
Fazio: Janseen: Honoraria. Caravita di Toritto: celgene: Other: travel expenses, Research Funding; Janseen: Other: travel expenses, Research Funding; amgen: Other: advisory board; takeda: Research Funding; GSK-SANOFI: Other: advisory board. Martelli: Gilead: Other: advisory board; Novartis: Other: advisory board. Petrucci: Janssen-Cilag: Honoraria, Other: Advisory Board; BMS: Honoraria, Other: Advisory Board; Takeda: Honoraria, Other: Advisory Board; Amgen: Honoraria, Other: Advisory Board; GSK: Honoraria, Other: Advisory Board; Karyopharm: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal